Tracking sperm over 50 years: From manual video micrography to cutting edge Computer Aided Sperm Analysis
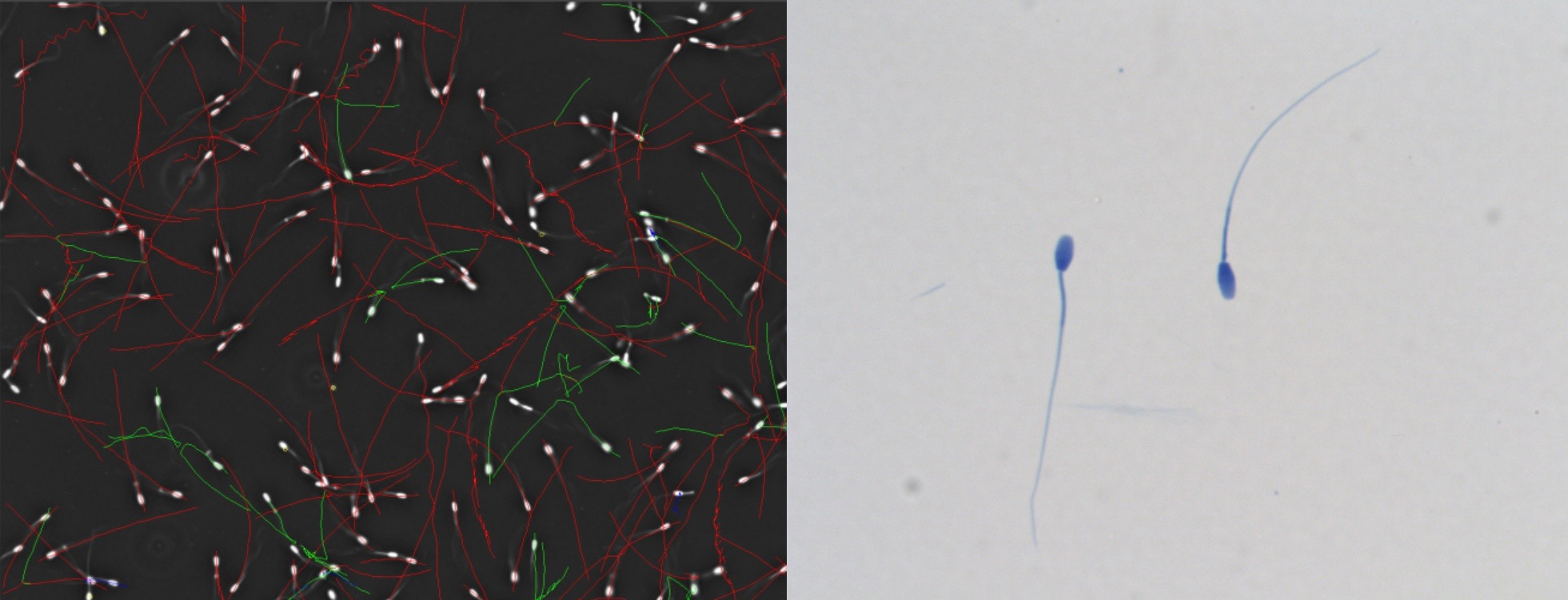
Currently, Computer Aided Sperm Analysis (CASA) systems are highly sophisticated and are used to evaluate sperm quality quantitatively by measurement of sperm concentration, motility, morphology, vitality, fragmentation, and the acrosome reaction among others. These aspects incorporate sperm functional analysis and some of these aspects relate to fertility outcome. Furthermore, the applications of “modern” CASA are vast and it is now routinely used in predominantly four fields: human andrology laboratories/IVF centres, domestic animal breeding centres including veterinary applications and wildlife, toxicology studies using mainly rodent sperm and for research in the previously mentioned applications. However, sperm quality quantification using predominantly CASA has a long history of more than 50 years.
The main purpose of this blog is to take you through this 50year journey of development and show how difficult a road it was to get to this era of modern CASA and effectively providing a solid quantitative scientific basis for evaluating sperm quality and replacing outdated and unreliable manual sperm analysis methods.
Motile sperm is a reflection of successful spermatogenesis, an intact and functional sperm cell membrane, mitochondrial function and overall biochemical soundness and has accordingly been regarded as one of the cornerstones of sperm quality assessment. It is accordingly not surprising that there have been many different approaches particularly in the 1970’s to quantify sperm motility. Attempts to measure sperm movement such as the remarkable efforts by Rothschild, Gray and Rikmenspoel in the 1950’s precede this but is outside the scope of this blog.
The approaches in the 1970’s focused on using video cameras with fast shutter speeds and in combination with stroboscopes track sperm movement. The best known studies among these were those of David Phillips, using the above techniques to photograph/videograph rat and mouse sperm. Using a Zeiss microscope with darkfield microscopy the camera shutter was opened for a second and stroboscope illumination from 50 pulses per second (Hz) to 500 Hz were applied. Afterwards the film was processed and the sperm images (head and tail) could be manually traced on the film and both sperm motility pattern and speed could be determined. Video-micrographic sperm tracking involved advancing the video frame by frame and then manually reconstructing the sperm tracks (Fig. 1, after Phillips, 1972).
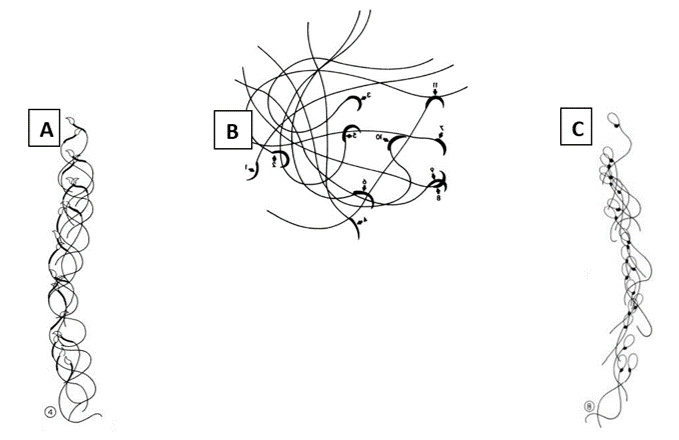
Fig. 1: Classical study of Phillips involving video-micrographic recording and manual tracking of (A) mouse, progressively moving sperm, (B) rat hyperactivated sperm and (C) human progressive sperm.
The advantage of this methodology was that both sperm head centroid movement as well as flagellar movement could be studied but the entire process was tedious, took several hours to trace a few sperm and was accordingly not practical.
However, the main theoretical, academic developments and practical applications to study sperm movement using video-cinematography took place in the famous laboratories of Prof Georges David in Paris and Dr Hector Dott in Cambridge during the 1970’s (Fig. 2). They were responsible for the development of kinematic parameters and not only the determination of the percentage sperm motility. David Katz and Jim Overstreet (Davis, CA, USA) then extended basic work on sperm
micro-cinematography. This was further pioneered by several including Samuels and van der Horst and was semi-automated with the programming skills of Hector Dott.

Fig. 2: Left Prof Georges David and on the Right Dr Hector Dott: the real pioneers of quantification of sperm motion and development of sperm kinematics in Paris and Cambridge respectively during the 1970’s. David’s sperm morphology classification system is still used in France and also implemented as an option of the SCA morphology classification system.
The basic video-micrography research on sperm motility of the 1970’s and early 1980’s paved the way for the development of CASA systems. Among the very first of these was the CellSoft system developed in the USA. While the system claimed accurate tracking and determination of the percentage sperm motility and sperm speeds, it was in essence a black box with no clear verification. From the late 1980’s there was an explosion in the development of CASA systems made possible by the advances in the use of personal computers. Among some prominent systems developed in the 1990’s were Hamilton Thorne, CellVision (USA), Cristmas, SpermVision, Androvision and SMQ. All systems made use of a video camera mounted on a microscope fitted with either positive or negative phase objective lenses and a computer with a specially designed graphics card and software. Analysis could be performed either directly or by playing recorded videos at frame rates varying from 25fps to 60fps. These systems have been extensively used and improved during the 1990’s and the early 2000’s and also for the first time operated in the very first Windows environments. Most of these systems could perform sperm concentration and sperm motility with detailed kinematics and also the percentage progressively moving sperm according to WHO criteria. In addition, many of the systems also provided a module for the quantitative analysis of sperm morphology. The Hamilton Thorne system provided the IVOS I during this period and it was the very first CASA system contained in one box with integrated optics and temperature control.
But the latest developments that cemented CASA as invaluable and essential in both the clinical and research settings during the last decade were mainly due to:
- Development of remarkable new computer hardware technology with 10th and 11th generation motherboards and solid state hard drives providing very fast personal computers typically with four processors and stacks of Rams. Furthermore, highly improved digital high resolution cameras that can routinely capture motile sperm at 25 to 200fps became available (made video cameras obsolete).
- Major new software developments including programming for artificial intelligence.
So, what have been new CASA developments in the last decade?
Many CASA developers and, in particular Microptic, changed the CASA scene by developing a suite of new CASA modules including vitality, fragmentation, acrosome reaction and leucocytes and improving many aspects of motility and morphology (Fig. 3). Accordingly, the emphasis now shifted to measuring many facets of sperm functionality such as hyperactivation, the ability to undergo the acrosome reaction and fragmentation which relates to fertilization outcome.
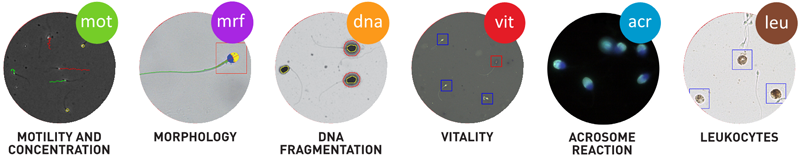
Fig. 3: The SCA suite of CASA modules enhancing analysis of sperm function.
Furthermore, Microptic developed the first fully automated CASA system (SCA SCOPE) (Fig. 4) contained in one box with integrated optics and temperature control but with the added advantage that analysis of prepared slides for motility, morphology, vitality, etc. takes place in 15 minutes in the absence of a technician. All completed analyses can be verified by a trained/qualified person.
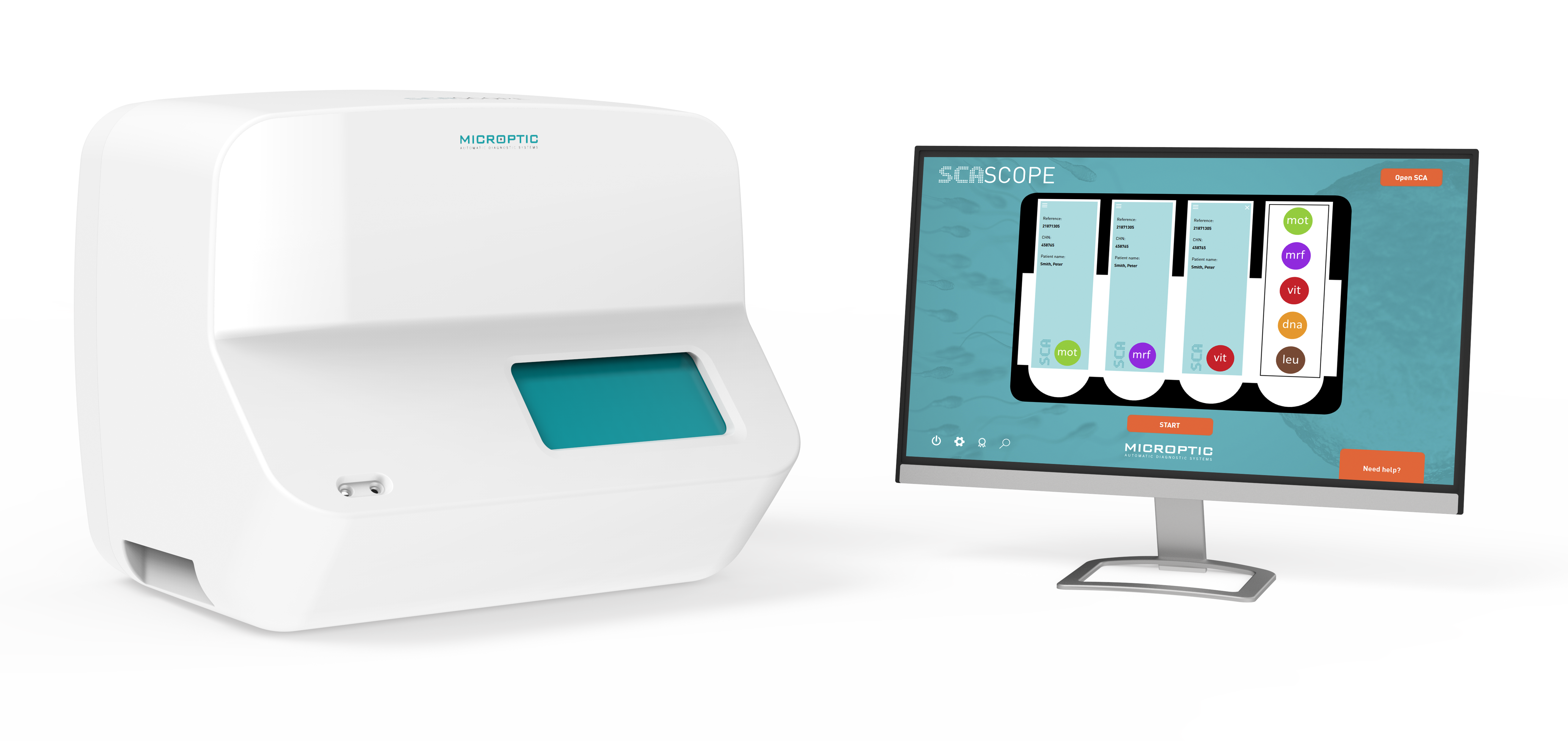
Fig. 4. The revolutionary SCA SCOPE, the first fully automated CASA system.
Further recent CASA developments concentrate on the importance of analysis of the flagellar beat at high frame rates (169fps). In this context the well-known group from the Univ. of Birmingham, UK, developed the FAST software (Flagellar and sperm tracking) (Fig. 5). It has the additional value of correlating head centroid analysis (current CASA systems) with the flagellar beat, the driving force of the sperm.
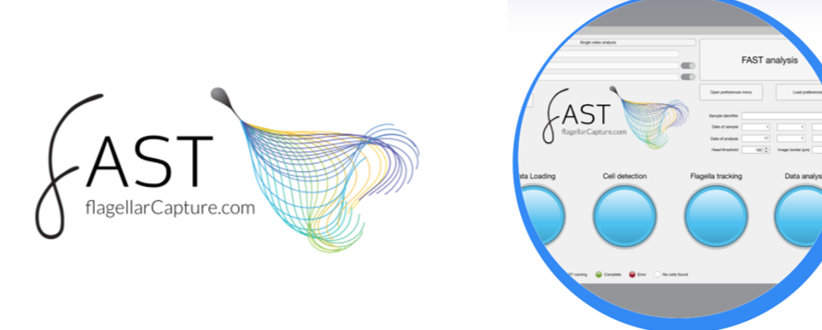
Fig. 5: Latest developments on automated analysis of the sperm flagellar beat at high frame rates.
Prof Gerhard van der Horst (PhD, PhD)
Senior Consultant
MICROPTIC S.L.




Leave A Comment